Probing the Secrets of the Universe from Snowden Hall
What happens to geological materials when a pressure equal to that of 880 African elephants standing on one square inch is applied to them? Sewanee planetary geologist Lily Thompson and her students are working hard to find out—and to expand our understanding of deep Earth, other planets, and beyond.
Sewanee’s Snowden Hall is a cabinet of wonders. Behold a cross-section of a redwood trunk, fossils embedded in the floor, and a paleontology laboratory with the imprint of ancient sea creatures and extinct plants. Assistant Professor of Geology Lily Thompson has made Snowden her professional home for the past two years, but the ancient past is not the only thing on her mind. Instead, Thompson and a group of energetic students are exploring a different kind of wonder. They are recreating conditions found deep in the interior of the Earth, on other terrestrial planets, and even within distant icy bodies, and their work is adding to humanity’s knowledge of how our planet, and other objects in the solar system, work.
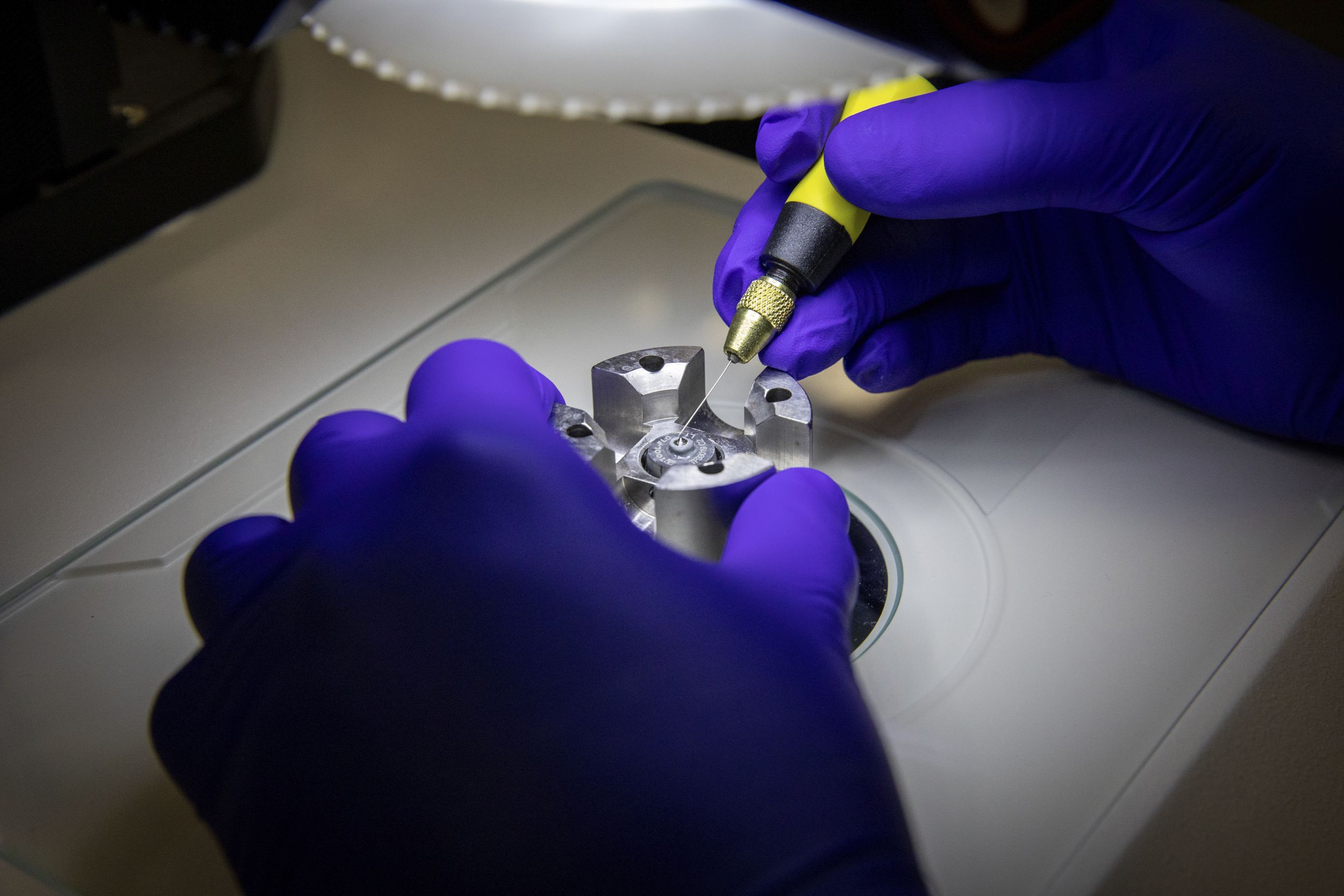
To recreate the extreme pressures of the Earth’s mantle in the laboratory, these researchers are using something called a diamond anvil cell (DAC), a device about the size of 10 air hockey pucks glued together—or, rather, two groups the size of five air hockey pucks (stainless steel ones at that) that are screwed together to replicate the pressure of 880 elephants per square inch.

Assistant Professor of Geology Lily Thompson
Assistant Professor of Geology Lily Thompson
Elephants?
Let’s explain. Geologists always have an Everest-sized hurdle in presenting their work. The Earth is so old that human beings have difficulty even conceptualizing just how old it is. Know, for example, that the Earth is 68,000 times older than the time that has elapsed between the meteor impact that wiped out the dinosaurs 66 million years ago and right now. Can you even?
Thompson has a separate conceptual challenge: how to explain conditions in one of her field sites, 1,600 kilometers (around 1,000 miles) under the surface of the Earth. For reference, the deepest hole human beings have ever dug is just over two kilometers deep, so Thompson is characterizing processes 800 times deeper. There, in the Earth’s mantle, the weight of all the kilometers of rock above creates intense pressure.
“In my field we have a measurement, the gigapascal [GPa] that no one understands, so I like to use elephants,” she says. “One GPa is the weight of 11 African male elephants standing on one square inch.” For one of Thompson’s projects, she and her students are studying how materials act in the Earth’s mantle at 80 GPa—in other words, the pressure that would be generated by the weight of 880 elephants stacked on top of a single square inch.
If actual elephants were in the laboratory, they would be standing on the DAC, which is the device that allows the research team to understand what is happening below our feet. At the center of the DAC are two tiny diamonds, cut so that each has a flat surface about the width of three human hairs. When the two halves of the diamond-containing DACs are squeezed together, an even smaller sample can be pressurized between the flat tips of the diamonds to generate immense pressures. The DAC assembly also includes a tiny ruby that is a 10th or 20th the diameter of a human hair that allows the scientists to monitor the pressure of the sample.
“You can put anything you want inside a DAC,” says Thompson. “We are probing geological materials so we can see how their physical properties change at high pressure.”
Wait. A ruby?
In short, the ruby is a diagnostic tool. The DAC has an opening through which a light can shine—a very bright light, in fact: a laser or something much brighter. Rubies fluoresce at a different wavelength (i.e., slightly different color) depending on temperature and pressure, so they act as a diagnostic. Researchers can look at the light spectra produced by a ruby within the sample chamber of a DAC and know the pressures and temperature at which the sample is being studied.
Over the past year, Thompson has worked with six students, three from the Physics Department and three from Earth and Environmental Systems, to build a ruby fluorescence system to help them understand what is happening inside the DAC. The device will hold a DAC, into which a blue-green laser is directed. The ruby will emit a bright red light, which is captured by a spectrometer, which provides a visual graph on an attached computer of the light spectrum and thus knowledge of what is happening within the DAC.

From left to right: Vincent DiNella, Alaa Adel Abdelhamid, Lily Thompson, Máté Garai, Audrey Burch, Christian Shushok.
From left to right: Vincent DiNella, Alaa Adel Abdelhamid, Lily Thompson, Máté Garai, Audrey Burch, Christian Shushok.
One of the principal areas Thompson is studying is how hydrogen is stored within the interior of the Earth. Recently, scientists in Thompson’s field have observed that the Earth has more water in its interior than on the surface in the form of oceans, rivers, marshes, glaciers, and clouds. We are not talking about underground lakes like in Jules Verne’s Journey to the Center of the Earth, but instead hydrogen and oxygen bonded to other materials in crystalline structures. According to Thompson, hydrogen in the deep Earth leads to profound effects, including volcanism, plate tectonics, rheology (how materials flow), and various physicochemical systems within the Earth.
“I was one of those irritating geology students,” says Thompson. “I would ask so many questions about what was happening at microscopic scales, and I would often reach a point where professors would dismiss the question by saying, ‘That’s something that happens at the atomic level.’ I decided that I really wanted to study what was happening at those fundamental scales.”
Two seniors, Alaa Adel Abdelhamid, C’22, and Vincent DiNella, C’22, took an early role in building the ruby fluorescence system, and also spent part of the summer working with a researcher on using another instrument, an electron microprobe, to analyze the minerals in meteorites.
“The composition of the samples gives us insight into the origin of the solar system,” says DiNella, showing slides of thin sections of materials that originated far away from Earth. The electron microprobe allows researchers to measure the presence of elements as small as a few parts per million. The students worked this summer to characterize the makeup of meteorites, which gives some understanding of those materials’ presence in the earliest solar system.
“As physics majors, this is really interesting work for us,” says Adel Abdelhamid. “It is really pure science, so it does not have an immediate use, but I think that gaining knowledge is itself the best application of research—satisfying our curiosity about the world."
While a functioning ruby fluorescence system is going to make pressure measurements possible for the Thompson group, the team will also have the opportunity later this semester to conduct research at Brookhaven National Laboratory’s synchrotron, a ring-shaped building in which electrons are being accelerated in a circle using magnets and then directed out as radiation in an experimental station. “There are many qualities of synchrotron radiation that make it an ideal analytical tool, such as stability and brilliance,” says Thompson. “These qualities allow us to focus on a really small sample and yet still collect meaningful measurements in a reasonable time frame.
Máté Garai, C’24, has been instrumental in building the ruby fluorescence system. He produced both a hand-drawn schematic and a more polished version that has guided how the team is assembling the important diagnostic device. “The system we built on campus will let us shine the sample with a laser with a known wavelength so that we can calibrate the DAC,” he explains. “That means when we go to Brookhaven we will know the exact pressure of our system.” Garai and others on the team are really excited about the trip to Brookhaven, and Thompson agrees that all the commotion at the laboratory makes it an exciting place, particularly for undergraduates, to have the opportunity to work. “My long-brewing dream is to visit a synchrotron and it looks like it could finally happen,” says Garai. “It will be an amazing experience and a huge honor to be able to visit a facility that contains the absolute peak of human scientific genius.”
Máté Garai's hand-drawn schematic of the ruby fluorescence system hangs in the laboratory ...
... where the system is being constructed.
Currently, the Thompson group is spending its time preparing the DACs and the experiments they will house. In the lab, Audrey Burch, C’24, and Christian Shushok, C’24, are working with steady hands at microscopes, cleaning the diamonds and preparing the DACs. Burch asks Thompson to inspect her work, and gets a nod of approval. “I’ve been cleaning this diamond for three weeks, and I am finally finished,” she says. “This is a terrifying but exhilarating experience,” adds Shushok.
“Typically, you might spend six months preparing the DACs,” says Thompson. Larger, more well-resourced universities might prepare as many as 30 loaded DACs for their 72 hours on the beamline, the end station guiding the synchrotron radiation at Brookhaven. The Thompson group will bring two, but they will also bring multiple prepared gaskets (each complete with its own sample and tiny ruby) so that they can reload DACs. “We get 72 hours, and we have to be working constantly during that time,” says Thompson. “Beamtime is expensive, so if we do not use our time productively, and if we do not get publishable results, then we may not be able to come back.”
The Thompson group looks at a variety of materials. For their work this fall, they will be looking at epsomite, a mineral that is found in some environments on the surface of Earth and Mars but that also occurs in icy bodies in the outer solar system. Thompson’s beamline proposal suggests two visits to Brookhaven to cover a large range of temperatures and pressures, from very cold to high-temperature observations.
Three hair's breadth
Thompson has been successful so far in producing beamline proposals and publishing the results of her work with colleagues from other universities including the University of Chicago, where she earned a Ph.D. In this relatively small field, she is something of a force herself, a power-nerd with a string of published peer-reviewed articles since 2011. She is associate editor of American Mineralogist, has won multiple fellowships, including a postdoctoral fellowship at Northwestern, and has chaired multiple sessions at professional conferences. But her path to this rarefied professional standing has been anything but straightforward.
“When I was in high school, I met with the guidance counselor, who said, ‘Lily, this will probably be our only meeting, because I don’t think you are college material.’” Thompson says. “I thought, OK, nice chat. What should I do now?” Soon she had settled comfortably into work as a hairdresser in Knoxville, where one of her clients was a nuclear physicist at Oak Ridge who had put herself through school as a hairdresser as well. That might have been her life for a while, but she developed a workplace repetitive-motion injury in her shoulder. “Too much blow-drying in the South,” she says. Having to take time off work, and inspired by conversations with her physicist client, Thompson decided to take classes at the University of New Orleans. “I started out as an education major, and I had to take at least one laboratory science course to get the degree. My advisor suggested geology, and I loved it.” Soon Thompson changed majors and plunged into geology, taking every course, asking all the tough questions of her professors, and applying for every research experience. Thompson is something of a poster child for a growth mindset. “Doing quantitative work in lab science wasn’t natural for me, but I figured out that it was a matter of practice.”
One of her research experiences as an undergrad was professionally adjacent to the work she does now, but she came within a hair’s breadth of another path. “I really wanted to do summer research with a group from the University of Texas involving caves, but I applied for every opportunity I could because I thought I was at a competitive disadvantage.” A week after she had accepted a place with a research group at the University of Minnesota on rock mechanics, the Texas professor reached out. “I didn’t want to change after accepting the position,” she says. “And I really loved the work with rock mechanics. It involved screws and wrenches and building understanding from the ground up, a grain of sand at a time. I really liked how systematic it was."
When longtime Sewanee Geology Professor Steve Shaver retired, the department set about finding someone who could teach mineralogy and petrology, his areas of specialty. “Those are courses you have to teach if you are offering a geology major,” Thompson explains. In finding that expertise, Sewanee has also discovered a planetary scientist of the first rank—a real rock star (as well as a rock squasher), an innovative and inspired teacher (who nimbly uses the illustrative power of elephants and human hair), and an accomplished mentor of undergraduate researchers.
